EXPERIMENTAL: SYNTHESES OF COMPOUNDS STRUCTURALLY RELATED TO CATALASE,
CHLOROPHYLL-a, HAEMOGLOBIN, and VITAMIN-B12
Introduction
In Nature, several dozen compounds contain the porphyrin skeleton, with
varying degrees of unsaturation, bonded to a wide range of functional
groups; examples of these so-called 'porphyrins' include: catalase, an
Fe(II) enzyme which catalyzes the decomposition of cellular hydrogen
peroxide; chlorophyll-a, one of several photosynthetic pigments which
transduce light into chemical energy in plants and other autotrophs;
haemoglobin, the Fe(II) pigment which is responsible for transporting
dioxygen and carbon monoxide in mammals; and vitamin-B12, the Co(III)
cofactor required for the synthesis of mammalian red blood cells.
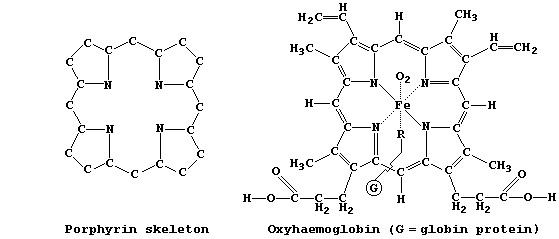
As hinted above, the structures of naturally occurring porphyrins are
very complex. So, in order to study some of their biological, chemical
and physical properties, scientists have synthesized 'simpler' versions
of these molecules; e.g., porphyrins A and B.
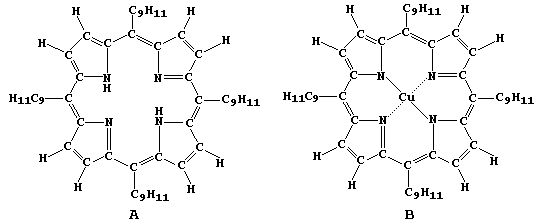
In this four-part series of experiments, you will synthesize copper
ethanoate, free-base porphyrin A, and copper(II) porphyrin B - from
porphyrin A and copper ethanoate - as well as examine porphyrins A
and B using chromatography. |
Method (Part 1) - Synthesis of Hydrated Copper(II) Ethanoate
1. Using a measuring cylinder, place 25 cm³ of dilute ethanoic acid
(1 mol dm-³) into a small beaker equipped with a stirring rod.
2. Warm the acid using a small Bunsen flame.
3. To the warm acid, add copper(II) carbonate in small portions until
in excess; i.e., until no more carbonate dissolves, there is no further
evolution of gas, and universal indicator paper no longer turns red.
4. Using a filter paper, filter the warm mixture; be sure to wash the
beaker and paper with a little distilled water.
5. Using a boiling water-bath, concentrate the copper(II) ethanoate
solution to about half its original volume.
6. Transfer the concentrated solution to a petri dish; then allow the
hydrated copper(II) ethanoate to crystallize at room temperature.
Method (Part 2) - Synthesis of Porphyrin A
1. Using a measuring cylinder, place 40 cm³ of propanoic acid into a
100 cm³ Pyrex beaker.
2. Using a hot plate contained in a fume-cupboard, carefully heat the
propanoic acid until it just starts to boil.
3. Using separate graduated (or automatic) pipettes, simultaneously
add pyrrole (1.0 cm³) and 4-isopropylbenzenecarbaldehyde (2.0 cm³).
4. Continue gently boiling this reaction mixture for 7 minutes; then
allow it to cool overnight.
5. Using a sintered-glass crucible, filter the mixture; then wash the
beaker and crystals with ethanoic acid (3 pasteur pipette measures),
followed by methanol (4 p.p.m.).
6. Dry free-base porphyrin A before placing it in a sample tube.
Method (Part 3) - Synthesis of Copper(II) Porphyrin B
1. Weigh about 0.10 g of porphyrin A into a small beaker; then add
trichloroethane (15 cm³).
2. Weigh about 0.10 g of hydrated copper(II) ethanoate into another
small beaker; then add methanol (15 cm³).
3. Using a hot plate (or a water-bath), separately heat both of the
above solutions until each is gently boiling.
4. Add the hot methanol solution to the trichloroethane solution; then
continue boiling for about 3 minutes.
5. Using a sintered-glass crucible, filter the mixture; then wash the
beaker and precipitate with warm methanol (3 p.p.m.), followed by warm
distilled water (4 p.p.m.).
6. Dry copper(II) porphyrin B before placing it in a sample tube.
Method (Part 4) - Chromatography of Porphyrins A and B
1. Using trichloroethane, freshly prepare separate solutions of both
porphyrins (A and B).
2. Develop the chromatograms of both porphyrins, using the same piece
of chromatography paper, in methanol:trichloroethane (5:1 ?).
Notes, Results (and Extension Work)
1. Copper(II) ethanoate crystallizes from an aqueous solution as the
mono-hydrate, Cu(CH3COO)2.H2O; i.e., copper(II) ethanoate-water(1/1).
2. Attach a sample of each compound and the best resolved chromatogram
to this worksheet, using Sellotape. Then, using a microscope, examine
the shapes and the colours (!) of your crystals.
3. Examine the catalytic behaviour, if any, of each compound; e.g.,
the decomposition of aqueous sodium chlorate(I), the decomposition of
aqueous hydrogen peroxide, the reaction of zinc with dilute sulfuric
acid, ... {Each porphyrin can be 'bulked-up' as follows: dissolve a
measured quantity of porphyrin in trichloroethane; stir in a measured
quantity of an 'inert' carrier [e.g., aluminium oxide, carbon-graphite,
or silicon(IV) oxide]; and then allow to evaporate to dryness.}
Dr. R. Peters Next Contents' List

