METALS: INTRODUCTION - REACTIVITY (1)
An element's metallic character can be precisely defined in terms of
electrical conductivity, as exemplified by the sub-set shown below:
[Ag > Cu > Al > Ca > Mg > Na > Zn > K > Fe > Sn > Pb > Hg]
However, an element's metallic character is more usually considered in
terms of chemical reactivity; and, as exemplified by the same sub-set,
there is no apparent correlation between conductivity and reactivity:
[K > Ca > Na > Mg > Al > Zn > Fe > Sn > Pb > Cu > Hg > Ag]
There is just the 'small' matter of defining chemical reactivity ...
Bon gré, mal gré?
The addition of a small quantity of solid potassium to water results in
the molten metal whizzing over the water's surface and the evolved gas
catching fire with a lilac flame. By contrast, a similar experiment with
solid lithium merely results in steady evolution of the gas (dihydrogen).
[Scene. A (kangaroo?) court in the state capital of Laputa]
Prosecutor: M'Lud. These results prove, beyond any reasonable doubt,
that Mr. Potassium is more reactive than the defendant.
Judge: Thank you kindly, Sir. [He addresses the prosecutor.] A
splendid case for the prosecution, if I may so say. Has
the defendant anything to add before I pass sentence?
Mr. Lithium: Yes, my Lord. I do admit that I have a higher activation
energy: but, my reaction with water is more exothermic
than Mr. Potassium's.
Judge: Stop! This Court will not be blinded by science ...
Mr. Lithium: But ...
Judge: Silence! You are in contempt of this Court. [He places
a black handkerchief over his grubby-looking, moth-eaten
wig.] Mr. Lithium, I sentence you, for the rest of your
natural life, to a position below Mr. Potassium in the
Reactivity Series. [Cheers from the public gallery.] |
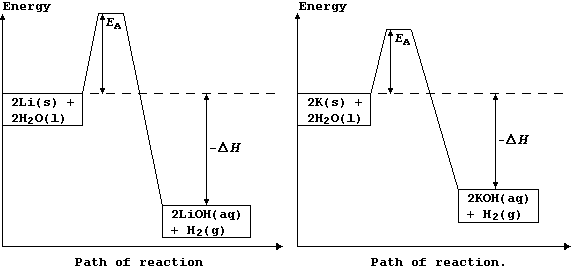
The explanation for the observations above is as follows. Potassium has
a low melting point, 63°C, and so the heat of reaction is sufficient to
make it melt; the molten metal spreads out to expose a larger surface
area, and so it reacts even faster: as a result, heating in situ causes
the dihydrogen gas to catch fire. By contrast, lithium reacts much more
slowly, because it has a higher melting point, 181°C, and so there are
fewer collisions between the particles; its reaction with water is more
exothermic, but this heat energy is released more slowly. These energy
level diagrams provide an alternative summary of this explanation.
[Scene. The re-trial in the same (kangaroo?) court of Laputa]
Counsel: M'Lud. These energy level diagrams are important new
evidence; they prove, beyond any reasonable doubt, that
my client is more reactive than Mr. Potassium.
Judge: Thank you most kindly, Sir. [He addresses counsel.] I am
obliged to say that you have presented a waterproof case
for the defence. [The legal beavers humour the judge by
smiling weakly.] A clear miscarriage of justice. Has the
defendant anything to add before I overturn the original
verdict?
Mr. Lithium: Indeed I do, my Lord. In contrast to Mr. Potassium, I
react readily with dinitrogen. Nevertheless, with most
other reactants, I am less ...
Judge: Please do stop! Time, or at least my time, is valuable.
[He glances briefly, but wistfully, at his golf clubs.]
This Court will not be blinded by yet more science ...
Mr. Lithium: But ...
Judge: Silence! You are in contempt of this Court. [He places
a white handkerchief over his (now) grubbier-looking,
moth-eaten wig.] Mr. Lithium, I place you in a position
above Mr. Potassium in the Reactivity Series. [Cheers
from the (clearly undiscriminating) public gallery.] |
Indirectly, the reactions of lithium and potassium 'crystallize' two
problems which bedevil chemists, whether they be putative or mature.
First, the almost irresistible tendency to blur the distinction between
the rate and the energy change of a reaction. This blurring appears to
establish strong roots at an early stage in one's scientific career: so
much so, that authors of university and specialist textbooks invariably
feel the need to remind their readers of the distinction. *
And second, perhaps in the desire 'to hammer the subject into shape', a
tendency to assume that a limited number of reactions will necessarily
establish a rule which can be applied willy-nilly (bon gré, mal gré).
Neither of these problems are likely to disappear in the near future:
but their adverse effects can certainly be minimized by bearing in
mind four principles.
First, there is no connection between the activation energy and the
heat energy change (DH) for a chemical reaction.
Second, to ensure consistency, chemical reactivities should be compared
only in terms of energy (DE) or heat energy (DH) changes. #
Third, despite inevitable limitations in its use, a (metal) reactivity
series based on standard redox potentials is inherently self-consistent
because it is derived from measured physical data.
And fourth, a metal (M) reactivity series, based on redox potentials in
aqueous solutions, and a Periodic Table of the Elements, which is just
a method of summarizing the ground-state electronic structures of
gaseous atoms, both provide a suitable focus for correlation: but,
neither should be considered as a substitute for experimental facts.
* See, for example, F. A. Cotton and G. Wilkinson, Advanced Inorganic
Chemistry, Wiley, New York, 1988; P. W. Atkins, Physical Chemistry,
Oxford University Press, Oxford, 1989; and, P. J. Sykes, A Guidebook
to Mechanism in Organic Chemistry, Longman, London, 1986.
# Acceptance of this principle does not preclude comparing the rates
of reactions. [In advanced studies, reactivities are compared in terms
of kinetic and thermodynamic stabilities, which are discussed in terms
of activation energies and free energy changes (DG), respectively.]
Dr. R. Peters Next Contents' List
