SELECTED PRINCIPLES: INTRODUCTION - ELECTRONIC STRUCTURES OF ATOMS
The ground-state electronic structure is the lowest-energy arrangement
of the electrons in each free, gaseous, neutral atom of an element.
Each neutral atom of an element consists of a nucleus of Z protons and
(A - Z) neutrons, together with Z extra-nuclear electrons; where Z is
the atomic number and A is the mass number. The extra-nuclear electrons
are arranged in atomic energy levels; the first four of these can hold
a maximum of 2, 8, 18, and 32 electrons, respectively. The ground-state
electronic structure of an atom is determined by a simple principle:
namely, electrons are arranged in such a way that their total energy is
a minimum. This Table shows two different methods of representing such
electronic structures for all 36 elements in Periods 1 to 4.
|
Occupancies of the
main energy levels |
Occupancies of the sub-levels of
the main energy levels (*) (†) |
Group |
ZAtom |
1st 2nd 3rd 4th |
1st 2nd 3rd 4th |
1 |
1H |
1 |
1s1 |
18 |
2He |
2 |
1s2 |
1 |
3Li |
2, 1 |
1s2, 2s1 |
2 |
4Be |
2, 2 |
1s2, 2s2 |
13 |
5B |
2, 3 |
1s2, 2s2 2p1 |
14 |
6C |
2, 4 |
1s2, 2s2 2p2 |
15 |
7N |
2, 5 |
1s2, 2s2 2p3 |
16 |
8O |
2, 6 |
1s2, 2s2 2p4 |
17 |
9F |
2, 7 |
1s2, 2s2 2p5 |
18 |
10Ne |
2, 8 |
1s2, 2s2 2p6 |
1 |
11Na |
2, 8, 1 |
1s2, 2s2 2p6, 3s1 |
2 |
12Mg |
2, 8, 2 |
1s2, 2s2 2p6, 3s2 |
13 |
13Al |
2, 8, 3 |
1s2, 2s2 2p6, 3s2 3p1 |
14 |
14Si |
2, 8, 4 |
1s2, 2s2 2p6, 3s2 3p2 |
15 |
15P |
2, 8, 5 |
1s2, 2s2 2p6, 3s2 3p3 |
16 |
16S |
2, 8, 6 |
1s2, 2s2 2p6, 3s2 3p4 |
17 |
17Cl |
2, 8, 7 |
1s2, 2s2 2p6, 3s2 3p5 |
18 |
18Ar |
2, 8, 8 |
1s2, 2s2 2p6, 3s2 3p6 |
1 |
19K |
2, 8, 8, 1 |
1s2, 2s2 2p6, 3s2 3p6 3d0, 4s1
|
2 |
20Ca |
2, 8, 8, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d0, 4s2
|
3 |
21Sc |
2, 8, 9, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d1, 4s2
|
4 |
22Ti |
2, 8, 10, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d2, 4s2
|
5 |
23V |
2, 8, 11, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d3, 4s2
|
6 |
24Cr |
2, 8, 13, 1 |
1s2, 2s2 2p6, 3s2 3p6 3d5, 4s1
|
7 |
25Mn |
2, 8, 13, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d5, 4s2
|
8 |
26Fe |
2, 8, 14, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d6, 4s2
|
9 |
27Co |
2, 8, 15, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d7, 4s2
|
10 |
28Ni |
2, 8, 16, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d8, 4s2
|
11 |
29Cu |
2, 8, 18, 1 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s1
|
12 |
30Zn |
2, 8, 18, 2 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2
|
13 |
31Ga |
2, 8, 18, 3 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p1 |
14 |
32Ge |
2, 8, 18, 4 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p2 |
15 |
33As |
2, 8, 18, 5 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p3 |
16 |
34Se |
2, 8, 18, 6 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p4 |
17 |
35Br |
2, 8, 18, 7 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p5 |
18 |
36Kr |
2, 8, 18, 8 |
1s2, 2s2 2p6, 3s2 3p6 3d10, 4s2 4p6 |
* The superscripts indicate the number of electrons in each sub-level;
for example, 3p5 means that there are 5 electrons in the 3p sub-level.
† Introductory courses invariably focus on the occupancies of the
main energy levels; so, the student is well advised to view all the
entries in this column as exotic species of tasty but unripened pears
(... which maybe either plucked at some later date or allowed to rot). |
The ground-state electronic structures of gaseous atoms, as summarized
by their occupancies of the main energy levels, can be represented by
simple electron-structure diagrams (as in the examples shown below).
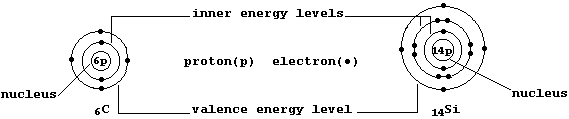
The Table shows the electronic structures of the first 26 elements
placed in the so-called main groups (i.e., 1, 2, and 13 to 18). Apart
from the single exception of helium, the elements in each group contain
the same number of valence (or bonding) electrons in their outermost
energy level; in part, it is this periodicity of electronic structures
which is the basis of the (modern) Periodic Table of the Elements.
The Table also shows the electronic structures of the first 10 elements
placed in Groups 3 to 12. Self-evidently, the occupancies of the two
outermost energy levels show no regularity; but, regrettably, there is
no simple explanation for the observed anomalies. Nevertheless, three
points are worth noting ... First, scandium (Group 3) behaves as if its
electronic structure was effectively 2,8,8,3; and so its chemistry has
some similarity to the elements in Group 13. Second, in contrast to the
main group elements, those in Groups 4 to 11, known as the transition
elements, have valence electrons in the two outermost energy levels or,
more specifically, in the 3d and 4s sub-levels. Because the necessarily
detailed descriptions of these sub-levels are (usually) reserved for an
advanced course, one's initial understanding of the chemistry of the
transition elements will inevitably be quite limited. And third, zinc
(Group 12) has two valence electrons in its outermost energy level; and
so its chemistry has some similarity to the elements in Group 2.
Currently, the received wisdom is that emphasis should be given to the
correlation of the periodicity of electronic structures with chemical
reactivity; an aspect which is readily achieved by limiting studies to
elements of Groups 1, 2, 17, and 18. However, many authors have drawn
attention to a plethora of caveats; just two of these are noted here. §
First, Cotton and Wilkinson have written: "Little of the chemistry of
silicon can be inferred from that of carbon." A particularly important
caveat when one reflects upon two observations: silicon is the second
commonest element in the Earth's crust, but carbon forms more compounds
than any other element apart from hydrogen. The following 'cluette' may
provide some perspective. A student, having compared the occupancies of
the main energy levels, would correctly reason that 'carbon and silicon
should show similar chemistry because they both have the same number of
valence electrons'. Contrastingly, a mature scientist, by drawing upon
advanced theoretical models, would reason that 'their chemistry should
differ because of the different characteristics of their occupied and
unoccupied sub-levels'. Thus, the two individuals will be perceiving
electronic structures (and the Periodic Table) in very different ways.
And second, elements in a group do not always form ions with the same
charge; e.g., in Group 11, the commonest ions of copper, silver, and
gold are, respectively, Cu(II), Ag(I), and Au(III) - admittedly, these
are transition metals: but then so are over half of all those known.
§ See, for example, F. A. Cotton and G. Wilkinson, Advanced Inorganic
Chemistry, Wiley, New York, 1988; and J. E. Huheey, Inorganic Chemistry
(Chapter 17), Harper Row, New York, 1983.
Dr. R. Peters Next Contents' List