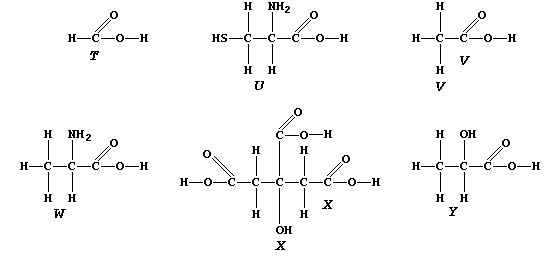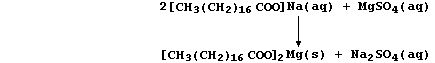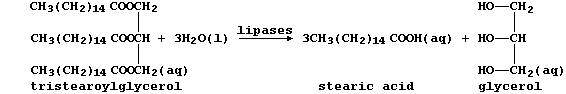ORGANIC CHEMISTRY: CARBOXYLIC ACIDS
The carboxylic acid functional group, -COOH, is present in a variety
of common chemical substances. This Table provides some information on
six such compounds; their structural formulae are shown below.
|
Systematic name / Common name |
Natural Occurrence |
|
Methanoic / Formic acid |
Wood ants (Formica rufa) |
|
2-Hydroxypropanoic / Lactic acid |
Sour milk / Active muscles |
|
2-Hydroxypropane-1,2,3-tricarboxylic
/ Citric acid |
Citrus fruits; e.g., sweet
oranges (Citrus sinensis) |
|
Ethanoic / Acetic acid |
Vinegar |
W |
2-Aminopropanoic acid / Alanine |
Via hydrolysis of proteins |
|
2-Amino-3-mercaptopropanoic acid /
Cysteine |
Via hydrolysis of proteins |
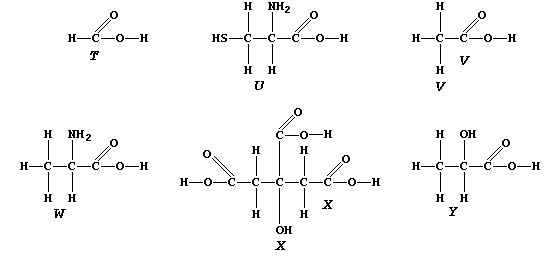
1. Complete the Table above, by reference to the systematic names and
structural formulae.
[5]
2. In contrast to 'mineral' acids [e.g., HCl(aq)], organic acids only
partially dissociate when dissolved in water; e.g., the position of
equilibrium lies far to the left in an aqueous solution of lactic acid: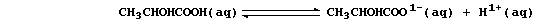
Although the lower concentration of hydrogen ions means that organic
acids react more slowly than mineral acids, reactions occur because -
as predicted by Le Chatelier's Principle - each equilibrium continually
'shifts to the right' as hydrogen ions are removed by reaction with
another reactant. Construct a symbol equation for the reaction between:
Magnesium and aqueous ethanoic acid ___________________________________
_______________________________________________________________________
Aqueous solutions of lactic acid and sodium hydroxide _________________
_______________________________________________________________________
Aqueous solutions of methanoic acid and potassium carbonate ___________
_______________________________________________________________________
Aqueous ethanoic acid and copper(II) oxide ____________________________
_______________________________________________________________________
[8]
Complete the following description of one method of preparing crystals
of hydrated copper(II) ethanoate (which has been used as a fungicide
and as an insecticide). "Wear _______ glasses. To 25 cmウ of warm - but
not boiling - ethanoic acid (1.0 mol dm-ウ), add copper(II) carbonate in
small portions until no more of this pale-green solid __________ (and
there is no more fizzing). Filter the mixture, partially __________ the
filtrate using a boiling water-bath, and then leave the dark-green
solution to crystallize at room temperature in a labelled petri dish."
[3]
3. One synthetic route to carboxylic acids involves oxidation of the
corresponding alcohol with acidified potassium dichromate(VI).
(a) When Acetobacter aceti bacteria use the ethanol present in wine as
a respiratory substrate, in order to biosynthesize ATP via aerobic
respiration, they excrete (sour-tasting) ethanoic acid:
Acetobacter aceti enzymes
C2H5OH(aq) + O2(g) 覧覧覧覧覧覧覧覧ョ CH3COOH(aq) + H2O(l) -DE
For the large-scale manufacture of ethanoic acid, suggest one advantage
in using a biotechnological process with these (immobilized) bacteria.
_______________________________________________________________________
[1]
Suggest the meaning of the term '- DE'. _______________________________
_______________________________________________________________________
[1]
(b) When Lactobacillus bacteria use the lactose present in milk as a
respiratory substrate, in order to biosynthesize ATP via anaerobic
respiration, they excrete (similarly sour-tasting) lactic acid:
Lactobacillus enzymes
C12H22O11(aq) + H2O(l) 覧覧覧覧覧覧覧覧ョ 4CH3CHOHCOOH(aq) -DE
Lactic acid is also formed, from glucose using different enzymes, when
a mammalian muscle carries out anaerobic respiration. The acid is toxic
to cells, and so must be oxidized back to glucose in the liver. How is
oxygen gas transported in the blood of a mammal? ______________________
[1]
4. This second Table provides some information on four of the main
naturally occurring, long-chain, saturated carboxylic acids (commonly
referred to as fatty acids because they were first isolated from fats).
Systematic name |
Common Source |
Condensed formula |
Tetradecanoic acid |
Coconuts (Cocus nucifera) |
CH3(CH2)12COOH |
Hexadecanoic acid |
Oil palms (Elaesis guineensis) |
CH3(CH2)14COOH |
Octadecanoic acid |
Cocoa seeds (Theobroma cacao) |
CH3(CH2)16COOH |
Eicosanoic acid |
Groundnuts (Arachis hypogea) |
CH3(CH2)18COOH |
(a) A typical soap is the sodium salt of octadecanoic acid, better
known as stearic acid, whose anions react with Group 2 cations present
in hard water to form a precipitate of 'scum'; e.g.,
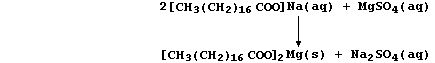
Noting that the spectator ions are aqueous sodium and aqueous sulfate,
construct the net ionic equation for this precipitation reaction. _____
_______________________________________________________________________
[2]
(b) The condensation of three fatty acids and glycerol, more correctly
known as propane-1,2,3-triol, results in a triglyceride. Fats and oils,
which are usually mixtures of triglycerides, are hydrolysed in mammals
by lipases, as this scheme exemplifies: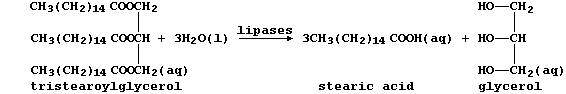
Several naturally occurring triglycerides contain unsaturated carbons.
Name one reagent which could show whether a fat or an oil was 'high in
polyunsaturates'. _____________________________________________________
[1]
Dr. R. Peters Next Contents' List