EXPERIMENTAL: AN INVESTIGATION INTO SOME OF THE VARIABLES INVOLVED IN
THE THERMAL CAPACITIES OF AQUEOUS SOLUTIONS
Introduction
Pure water is a covalently bonded compound with some unusual physical
properties; these include a high boiling point, a high surface tension,
a maximum density as a liquid, a high latent heat of vaporization, and
the highest thermal capacity of any liquid.
However, pure water is rarely encountered in Nature because it is such
an excellent solvent in which many solutes dissolve to varying degrees.
Furthermore, the resulting aqueous solutions might quite reasonably be
expected to have different properties; e.g., thermal capacities.
A detailed knowledge of variables involved in the thermal capacities of
aqueous solutions should be important in order to understand a variety
phenomena, such as 'homeostatic control of temperature within cells',
or 'the effect of global warming on aquatic organisms living in saline
environments', or 'the use of sea water as an external coolant', or ...
In this investigation, you are required to examine at least three
variables involved in the thermal capacities of aqueous solutions;
at least one of these must be quantitative, and at least one must
be qualitative. |
Notes
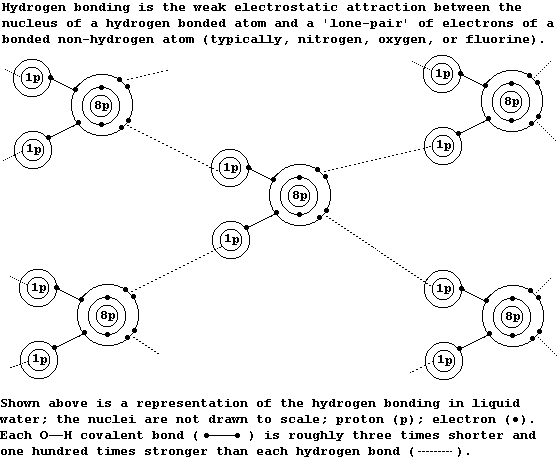
1. All of these properties of water are partially attributable to its
extended structure, which involves 'hydrogen bonding'; although many
molecules show hydrogen bonding, (including ammonia, DNA, ethanol,
propane-1,2,3-triol, and most enzymes), each water molecule is unique
in its ability to hydrogen bond with four other water molecules.
2. Apart from your notes and standard textbooks, sources of scientific
knowledge include encyclopaedias in libraries, on CD-ROMs, and on the
Web; it is good practice to include a bibliography in your write-up.
3. You are provided with a variety of compounds, distilled water, a
joulemeter, and a direct current supply. In addition, you will need to
use - within reason - other suitable apparatus.
4. The proposed plans of your investigation should be presented in
detail; these plans may be modified as the investigation proceeds.
Dr. R. Peters Next Contents' List
