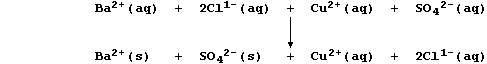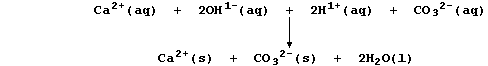METALS: PRECIPITATION REACTIONS
A precipitation reaction is the chemical change which occurs when two
ionic reactants give an insoluble product from an aqueous solution;
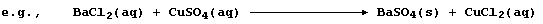
Because an ionic compound dissociates into (hydrated) cations and
anions when dissolved in water, a better perspective of a precipitation
reaction is gained by constructing an ionic equation; i.e.,
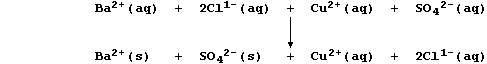
Those ions that are not involved in the overall reaction, here Cu2+(aq)
and Cl1-(aq), are referred to as spectator ions; and, so as to focus on
the change that actually occurs, a net ionic equation is often written;
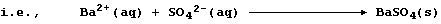
In order to be able to predict whether a precipitate will be formed
when two aqueous solutions are mixed, one needs to know the solubility;
i.e., the maximum amount of solute that can be dissolved in the solvent
water at a specific temperature. Extensive compilations of solubility
data are available in reference books (e.g., The Handbook of Physics
and Chemistry), though it is common practice to divide compounds into
three broad categories based on their solubilities in water at 20°C;
i.e., 'soluble' (> 10 g kg-¹), 'slightly soluble' (1 - 10 g kg-¹), and
'insoluble' (< 1 g kg-¹). These categories are then used to formulate
'solubility rules' - as exemplified by A ————® F.
A. All Group 1 and ammonium compounds are soluble.
B. All nitrates, hydrogencarbonates, and ethanoates are soluble.
C. Most chlorides, bromides, and iodides are soluble; the exceptions
are those containing silver(I) or lead(II) ions.
D. Most sulfates are soluble; CaSO4, SrSO4, and Ag2SO4 are slightly
soluble, whereas BaSO4 and PbSO4 are insoluble.
E. Most carbonates, phosphates, and sulfides are insoluble; the
exceptions are those containing Group 1 or ammonium ions.
F. Most hydroxides are insoluble; Ca(OH)2 and Sr(OH)2 are slightly
soluble, whereas Ba(OH)2 and Group 1 hydroxides are soluble.
1. Probably the most familiar example of a precipitation reaction is
that observed in the limewater test for carbon dioxide; the formation
of the characteristic milky-white precipitate is usually summarized as:
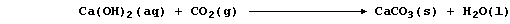
However, this symbol equation clearly requires explanation ... The gas
carbon dioxide, a covalently bonded compound, dissolves slightly in
water to form carbonic acid, H2CO3(aq), which partially dissociates;
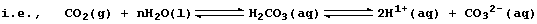
Le Chatelier's Principle predicts that both equilibria will shift to
the right if aqueous carbonate ions are removed from solution; and, as
the ionic equation below shows, this will occur by precipitation:
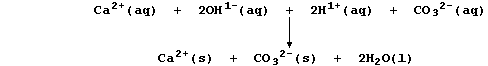
(a) This ionic equation reveals a precipitation and a neutralization
reaction. Construct a net ionic equation for each reaction. ___________
_______________________________________________________________________
_______________________________________________________________________
[2]
(b) Continued bubbling of carbon dioxide through limewater results in
the (initially formed) precipitate redissolving. Construct the symbol
equation for this reaction. ___________________________________________
_______________________________________________________________________
[2]
2. The precipitation reaction between aqueous solutions of potassium
iodide and lead(II) nitrate was investigated quantitatively.
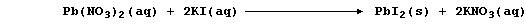
Using a pipette, 10.0 cm³ of aqueous lead(II) nitrate (0.100 mol dm-³)
was added to a test tube, and then, using a burette, 3.0 cm³ of an
aqueous solution of potassium iodide was added. The reaction mixture
was shaken thoroughly, and then, in the absence of a centrifuge,
allowed to stand for 1 hour before the height of the yellow precipitate
was measured (to within 0.5 mm). This procedure was repeated several
times, except different volumes of aqueous potassium iodide were used;
the Table shows a summary of the results.
Vol. KI(aq)/cm³ |
0.0 |
3.0 |
6.0 |
9.0 |
12.0 |
15.0 |
18.0 |
21.0 |
24.0 |
27.0 |
Ht. ppte./mm |
|
4.5 |
8.0 |
11.5 |
16.5 |
20.0 |
24.0 |
27.0 |
27.5 |
27.0 |
(a) Insert the missing value in the above Table, and then plot all ten
data points. _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
27_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
24_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
21_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
Height of 18_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
precipitate 15_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
/ 12_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
mm 9_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
6_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
3_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
0_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|_|
| | | | | | | | | |
0 3 6 9 12 15 18 21 24 27
Volume of KI(aq) / cm³
[3]
(b) Draw the two best straight lines, and then, by intersecting these
lines, determine the minimum volume of aqueous potassium iodide
required to react completely with 10.0 cm³ of aqueous lead(II) nitrate
(0.100 mol dm-³). _____________________________________________________
[3]
(c) Use this value, and the symbol equation, to state the concentration
of the aqueous potassium iodide solution used in this investigation.
_______________________________________________________________________
[1]
3. Soluble lead(II) ions, which inhibit the active sites of a number
of enzymes, are absorbed by diffusion across semi-permeable membranes;
so, soluble lead(II) nitrate is obviously more toxic than any insoluble
lead(II) compound. Nevertheless, the accidental ingestion of aqueous
lead(II) nitrate is rarely fatal, in humans at least, because insoluble
lead(II) ions are precipitated from the reaction between this solution
and the hydrochloric acid present in gastric juice. Construct both the
symbol and net ionic equations for the precipitation reaction between
aqueous solutions of lead(II) nitrate and hydrochloric acid. __________
_______________________________________________________________________
_______________________________________________________________________
[3]
Dr. R. Peters Next Contents' List